Most people quite well imagine iron and aluminum, silver and gold. But there are chemical elements that play a slightly smaller role in the lives of the modern world, but undeservedly little-known among non-specialists. It is important to correct this drawback, and including learn everything about Iridia.


Peculiarities
Immediately it is worth saying that Iridium is a metal. Therefore, it has all those properties that are typical for other metals. Such a chemical element denoted by the combination of Latin IR characters. In the Mendeleev table he takes 77 cell. The opening of Iridia occurred in 1803, in the framework of the same study, in which the English scientist Tennant allocated the Osm region.
The initial raw materials for the production of such elements served ore platinum delivered from South America. Initially, the metals were allocated in the form of a sediment, which "did not take" the "tsarist vodka". The study showed the presence of several previously unknown substances. The element received his verbal designation because his salts look like a releasing rainbow.
The content of iridium in nature is exceptionally small, and this is one of the rarest substances on Earth.


Chemically pure iridium has no rainbow color. But for it, quite attractive silver-white color is characteristic. Toxic properties are not confirmed. However, individual compounds of iridium may be dangerous to humans. Especially poisonous fluoride of this element.
A number of Russian and foreign enterprises are engaged in the production and affination of Irida. Almost the entire production of this metal is a product of platinum raw materials. Although Iridium and not purple, it contains in natural form 2 isotope. 191st and 193th elements are stable. But pronounced radioactive properties of but has a number of artificially obtained isotopes, their half-life is small.

Properties
Physical
The strength and hardness of Iridia is very high. It is almost impossible to mechanically process this metal. Infusibility This element of silver-white color is large enough. Specialists Believe iridium to the platinum group. The hardness on the MOOS scale is 6.5. The melting point in degrees reaches 2466 degrees. Boiled Iridium, however, starts only at 4428 degrees. The heat of melting is equal to 27610 J / mol. The heat of boiling is 604000 J / mol. The molar volume of specialists determined at the level of 8.54 cubic meters. See mole.
The crystal lattice of this element is cubic, the vertices of the cube are the face of crystals. The fraction of the 191st isotope accounts for 37.3% of Iridia atoms. The remaining 62.3% are represented by the 193rd isotope. The density of this element (or otherwise, the proportion) reaches 22,400 kg per 1 m3.
In its pure form, the metal is not a magnetic, and the degree of oxidation of atoms in various connections ranges from 1 to 6.


Chemical
But the Iridium atoms themselves rarely enter into any reaction. This element is distinguished by an outstanding chemical passivity. . It does not completely dissolve in water and does not change in some way, even with long-term contact with air. If the temperature of the substance is less than 100 degrees, then it will not enter into the reaction even with the "royal vodka", not to mention other acids and their combinations. The reaction with fluorine is possible at 400 degrees, for the reaction with chlorine or sulfur, you will have to warm the iridium to red cagine.
4 chloride are known in which the number of chlorine atoms varies from 1 to 4. The effect of oxygen is noticeably at a temperature not lower than 1000 degrees. The product of such interaction is iridium dioxide - a substance practically insoluble in water. It is possible to increase solubility by oxidation using the complexing agent. The highest degree of oxidation in normal conditions can only be achieved in Iridium hexafluoride.


At extremely low temperatures, compounds with valence 7 and 8 appear. It is possible the formation of complex salts (both cation and anionic type). It is noted that a strongly preheated metal can eliminate hydrochloric acid saturated with oxygen. An important role of chemists is attached:
- hydroxides;
- chlorides;
- halides;
- oxide;
- Carbonilas Iridia.


How to mining?
Obtaining iridium in nature is very difficult to be very rare. In a natural medium, this metal is always mixed with concomitant substances. If this element is detected somewhere, then platinum or metals from its group are necessarily located nearby. Some ores containing nickel and copper include iridium in scattered form. The main part of this element is extracted from oblique matter in:
- SOUTH AFRICA;
- Canada;
- North American California state;
- deposits on the island of Tasmania (owned by the Australian Union);
- Indonesia (on the island of Kalimantan);
- Different regions of New Guinea island.

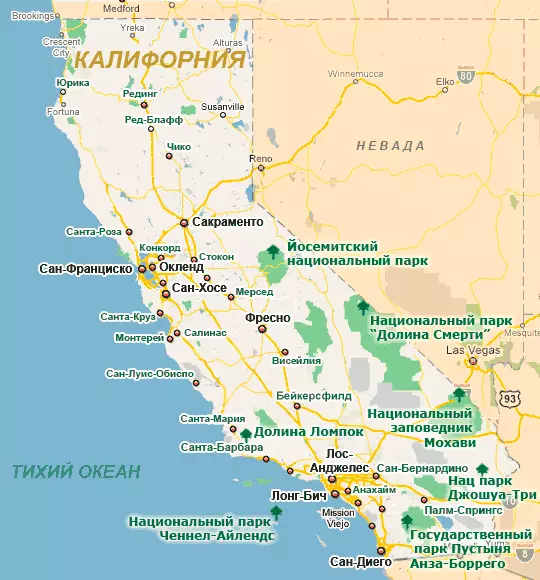
Mixed with Osmimia Iridium is mined in old mountain collections located in the same countries. The dominant role in the global market is occupied by companies from South Africa . It is not for nothing that the development in this country directly affects the balance of demand and suggestions, which cannot be said about products from other regions of the planet. According to existing scientific ideas, the rarity of iridium is connected with the fact that it fell to our planet only in meteorites, and therefore it accounts for a million percentage of the percentage of the mass of the earth's crust.
However, part of the experts do not agree with this. They insist that only a small part of all Iridia deposits are explored and is suitable for developing at the level of modern technologies. The deposits that appeared in deep geological antiquity contain in separate layers of iridium hundreds of times more than the breeds already developed.
Such anomalies are found on the whole globe. However, the extraction of material from deep cuts under the mainland and at the bottom of the oceans is still economically irrational.


Today Iridium is mined only after the end of the mining of the main minerals . This is gold, nickel, platinum or copper. When the field is close to exhaustion, the ore begins to process with special reagents that release rutheniums, osmium, palladium. Only after them comes a queue of the "rainbow" element. Further:
- Clean the ore;
- crush it into powder;
- Put this powder;
- Interpret compressed blanks in electrical furnaces, with continuous argon jet movement.
A sufficiently large amount of metal is extracted from the anodic sludges left by copper-nickel production. Initially, the sludges enriched. Transfer into a solution of platinum and other metals, including iridium, occurs under the action of hot royal vodka. Osmis turns out to be in a unwanted sediment. From the solution under the action of ammonium chloride, platinum, iridium and ruthenium complexes are consistently deposited


Application
About 66% of the mined Iridia used in the chemical industry . All other sectors of the economy share the balance. In recent decades, the jewelry meaning of the "purple metal" is growing steadily . Since the end of the 1990s, rings, inlaying gold jewelry began to produce from it. Important: Jewelry makes not so much of pure iridium, how much of its alloy with platinum. A 10% supplement is enough to improve the strength of the workpiece and the finished product to increase without a significant increase in cost up to 3 times.
In other industries, iridium alloys are also definitely ahead of pure metal. The ability to increase the hardness and strength of products by insignificant additive is very valued by technologists. So, iridium additives are used to increase the wear resistance of the wire for electronic lamps. The solid metal is simply imposed on top of molybdenum or tungsten. The subsequent sintering occurs under the press at high temperature.


And here it is necessary to specifically say about the use of iridium in the chemical industry. There it is needed to get the alloys to get the dishes resistant to different reagents and high temperatures. Also, iridium turns out to be an excellent catalyst. Increased reaction capacity especially manifested in the production of nitric acid . And if you need to dissolve gold in the royal vodka, then the technologists are almost guaranteed to choose exactly the bowls made from platinum-iridium alloy.
Where they cook Laser crystals often you can meet Platinum-iridium crucible. Fully pure metal is suitable for parts of particularly accurate industrial and laboratory devices. Mouthpiece from iridium is used and Glaziers When they need to make refractory glass grade. But it is only a small part of the applies of an amazing element.
It is often used in the manufacture of spark plugs for cars.


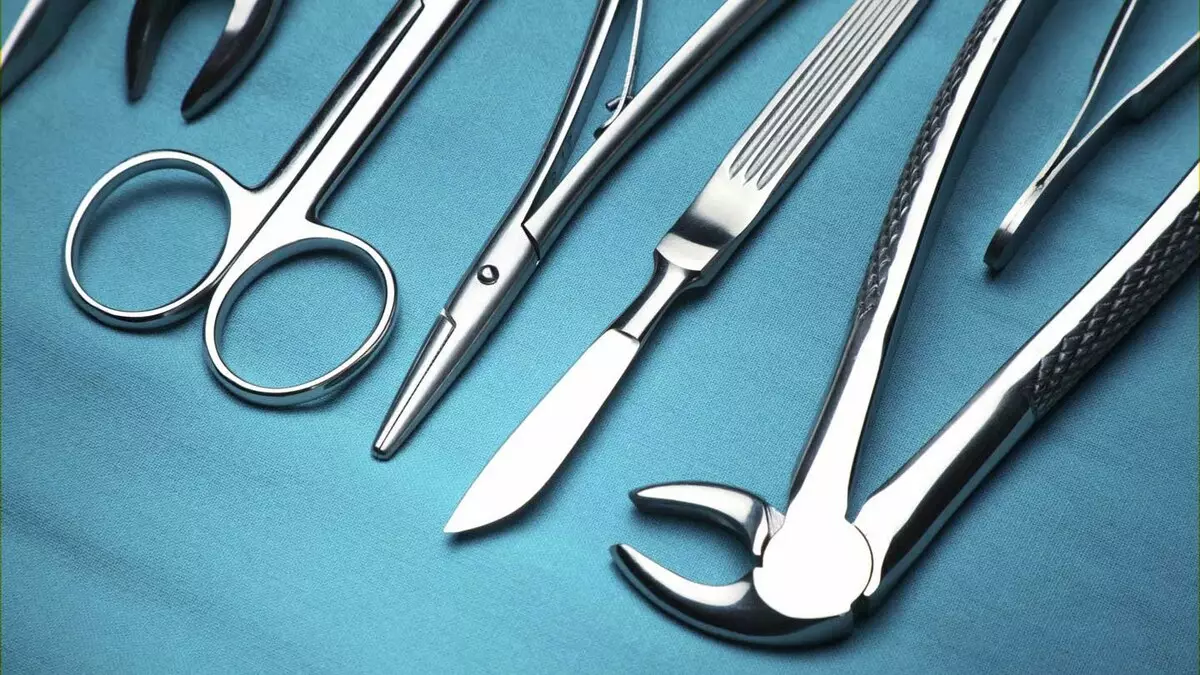
Experts have long noted that such candles serve longer . At the very beginning they were used mainly for sports cars. Today they became cheaper and turned out to be available to almost all car owners. Iridium alloys are also needed by the creators Surgical instruments . Increasingly, they are also used in the production of individual parts of the pacemaker.
It is curious that the "10 francs" coin of the production of Rwanda is made from the jewelry of the pure (999 sample) of Irida. Finds this metal application in automotive catalysts. Like platinum, it helps to accelerately clean the exhaust gases. But it is possible to find iridium in the most ordinary feathers handle. There, sometimes you can see the ball of unusual color, located on the tip of the pen or ink rod.

In radio components, Iridium used mostly a few decades ago. From it, contact groups were made more often, as well as components that can be very hot. Such a solution allows for the durability of products. Iridium-192 isotope refers to the number of artificial radionuclides. It is made for flaw detection to use the characteristics of welds, steel and aluminum alloys.
Osmia alloy with iridium apply to make Compass needles. And thermocouples in which iridium and conventional electrodes are combined, are used for physical research. Only they can directly register a temperature of about 3000 degrees. The price of such structures is very large. Use them in the usual industry is not economically impractical.


Iridiyevo titanium electrode - One of the relatively new developments in the field of electrolysis. The refractory substance is spoken based on titanium foil. In the working chamber, there is only argon. Electrodes may look like a grid, and as a plate. Such electrodes:
- high temperature resistant;
- resistant to significant voltage, density and current strength;
- do not corrod;
- more economical electrodes with platinum additive (due to a significantly higher resource).

Low-stranded containers with radioactive isotopes Iridia are in demand in metallurgy. Gamma rays are partially absorbed by the mixture. Therefore, it is possible to determine what the level of the mixture inside the furnace.
You can still specify the applications of the 77th element as:
- obtaining molybdenum and tungsten alloys, stronger at high temperatures;
- increasing the durability of titanium and chromium to acids;
- production of thermoelectric generators;
- manufacture of thermionic cathodes (along with lanthanum and cerium);
- creation of fuel tanks for space missiles (in alloy with hafnia);
- Development of propylene on the basis of methane and acetylene;
- Additive to platinum catalysts to produce nitrogen oxides (nitric acid precursors) - but this technological process is no longer too relevant;
- Obtaining reference units of measurement (more precisely, it is necessary for a platinum-iridium alloy).

Interesting Facts
Iridium salts are very diverse in color. So, depending on the number of attached chlorine atoms, the compound may have copper-red, dark green, olive or brown colors. Iridium difluoride is painted in yellow tone. Connections with ozone and bromine have blue color. In pure iridium, corrosion resistance is very large even when heated to 2000 degrees.
In the rocks of earthly origin, the concentration of iridium compounds is very small . It is seriously rising only in the breeds of meteoric origin. Such a criterion allows researchers to establish important facts about various geological structures. In total, only a few tons of iridium produced on Earth.
Jung module (it is a module of longitudinal elasticity) in this metal - in second place among well-known substances (more - only in graphene).


For other properties and spheres of Iridia, see the following video.
